Hydrofluoric Acid and Water Chemical Equation
But oxygen in the water is oxidized to ozone O 3. Write the equation for reaction of hydrofluoric acid and its conjugate base fluoride ion with water.
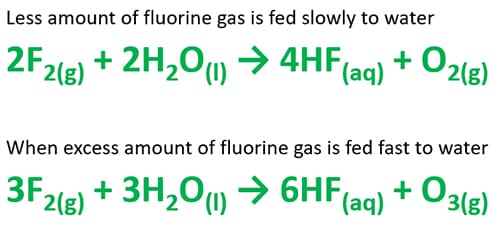
Fluorine And Water Reaction F2 H2o
4 Anhydrous hydrogen fluoride is one of the strongest acids known.

. What is Hydrofluoric Acid. It is used to make most fluorine-containing compounds. Hydrogen fluoridehydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil.
It is a chemical compound and considered a. This equation mE be converted to a linear form by multiplying by y C adding and subtracting C Ao YK k to the right side dividing by 1 C k 12 squaring both sides and simplifying. 4 moles of hydrofluoric acid reacts with 1 mole of silicon dioxide to produce 1 mole of silicon tetrafluoride and 2 mole of water.
4 Hydrogen fluoride is a colorless gas that is highly soluble in water. This procedure 1 gives. 4HFaq SiO2 s.
The chains in the molecules are usually shorter and exist between five and six molecules on an average. Hydrofluoric acid is a solution of hydrogen fluoride in water. In this video well write the correct formula for Hydrofluoric acidTo write the formula for Hydrofluoric acid well use the Periodic Table and follow some.
Elemental fluorine is produced from it. Hydrogen fluoride mixes readily with water forming hydrofluoric acid. The chemical equation for the reaction of two follows.
HFaq H2Ol H3O aq F aq By definition the acid dissociation constant for this equilibrium will be. Hydrofluoric acid is a solution of hydrogen and fluoride mixed with water. According to the above discussion we conclude the balanced equation for hydrofluoric acid HF reacts with silicon dioxide to produce silicon tetrafluoride and water is 4HF SiO_2 to SiF_4 2H_2O Note.
Hydrofluoric Acid Chemical Formula. That is silicon tetrachloride. And the products of the reactions are silicon tetrachloride.
Furthermore this acid is a starting compound for almost all the fluorine-containing compounds. In this video we will look at the equation for HF H2O and write the products. Hydrofluoric acid is hydrogen fluoride in water.
Say for silicon to travel right. Hydrofluoric acid is a solution of hydrogen and fluoride mixed with water. According to the Bronsted Lowry concept Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
The state is um S. 9 The term 1-Ao Ao 2 1 kC becomes negligible at. This acid is highly reactive with glass and moderately reactive.
HFaq SiO2 s SiF4 g H2OL To balance this equation all you have to do is multiply the hydrofluoric acid by 4 and the water by 2. Lets discuss the question. It is a chemical compound and considered a.
The balanced equation is Explanation. The balanced chemical reaction will be In this reaction hydrofluoric acid is act as a Bronsted. This is a good example for water is oxidized to oxygen gas reaction.
The equilibrium that is established when hydrofluoric acid ionizes looks like this. The Hydrofluoric acid is a strong acid but it is a weak electrolyte hence when hydrofluoric acid is dissolved in the water it produces ions and some of the molecules of this acid in the water solution. The odor threshold is 003 mgm 3.
CA2AijK 2AoAo-l 1-AoAo21 kC AijKC k. A a Ka F H3O HF a a. It is written as HF in chemistry and its molecular mass is approximately 2001 gmol.
It is commonly used to etch glass and silicon wafers. 3F 2 3H 2 O 6HF O 3. Show that the equations will give the auto ionisation equilibrium for water and that Ka x Kb Kw.
Solutions of HF are colourless acidic and highly corrosive. For all practical purposes they are considered the same chemical. By Stoichiometry of the reaction.
The chemical formula of hydrofluoric acid is given as HF. Hydrogen fluoride is a weak acid. Hydrogen fluoride is given in this reaction too.
This will ensure that all the atoms present on the reactants side are accounted for on the products side. In some cases we are not able to attain the balance by changing the mole. It is an intermediate for many chemical reactions and syntheses.
Its chemical formula is HF. The acid consists of a diatomic molecule with strong intermolecular hydrogen bonds. Hydrogen fluoride is an acidic compound having the chemical formula HF and molar mass 20 gmol.
Excess amount of fluorine gas is fed fast to water this will give hydrogen fluoride and ozone. When we add HF to H2O the HF will dissociate and break into H and F-. Hydrofluoric acid HF is a popular aqueous solution prepared from the hydrogen fluoride and other popular name for this chemical substance is fluoric acid.
4 Hydrogen fluoride has a strong irritating odor. Its chemical formula is HF. And Water Plus H 20.
In balanced chemical reaction wise we predict the side product of the reaction. Hydrofluoric acid chemical formula HF is a solution of anhydrous hydrogen fluoride in water typically 25 38 or 40 49 70 or 79 and is the form usually shipped by railcar or in smaller tanks and is the form usually stored at a plant site. Formula of Hydrofluoric acid HF is used to each glass.
The chemical formula for hydrogen fluoride is HF and its molecular weight is 2001 gmol. The question is Hydrofluoric acid. Examples include the commonly used pharmaceutical antidepressant medication fluoxetine and the material PTFE.
Thus when hydrofluoric acid is dissolved in the water solution is major species and are and minor species present in solutions. It is also available in the gaseous form and usually mixed with the water. Write the balanced chemical equation.

Hydrofluoric Acid Formula Structure Preparations And Properties
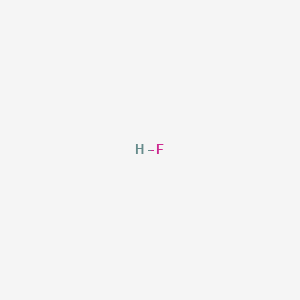

Comments
Post a Comment